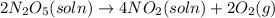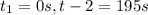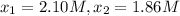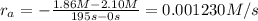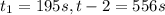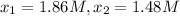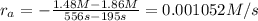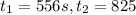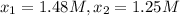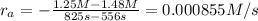The decomposition of n2o5 can be described by the equation.
2n2o5 (soln) > 4no2 (soln) + 2...

Chemistry, 07.07.2019 03:10 natalie2sheffield
The decomposition of n2o5 can be described by the equation.
2n2o5 (soln) > 4no2 (soln) + 2 (g)
given this data for the reaction at 45 degrees c in carbon tetrachloride solution, calculate the average rate for each successive time interval.
t(s) [n2o5] (m)
0 2.10
195 1.86
556 1.48
825 1.25
interval: 0 s to 195 s
reaction rate= /s
195 s to 556 s
reaction rate= /s
556 s to 825 s
reaction rate= /s

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 04:10
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1
You know the right answer?
Questions




English, 02.08.2019 02:30

Physics, 02.08.2019 02:30




Computers and Technology, 02.08.2019 02:30


Mathematics, 02.08.2019 02:30

Computers and Technology, 02.08.2019 02:30

Social Studies, 02.08.2019 02:30

History, 02.08.2019 02:30



French, 02.08.2019 02:30

English, 02.08.2019 02:30


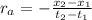
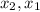 : concentration at time
: concentration at time 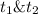 respectively.
respectively.