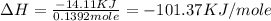Chemistry, 06.07.2019 19:10 angelZ3947
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g.
a. -101.37 kj
b. -7.05 kj
c. 7055 kj
d. 10,1365 kj

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2


Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was ne...
Questions


Mathematics, 22.03.2021 17:30


Mathematics, 22.03.2021 17:30



Mathematics, 22.03.2021 17:30

Mathematics, 22.03.2021 17:30

Mathematics, 22.03.2021 17:30


Health, 22.03.2021 17:30



English, 22.03.2021 17:30


History, 22.03.2021 17:30

English, 22.03.2021 17:30


Biology, 22.03.2021 17:30

Mathematics, 22.03.2021 17:30

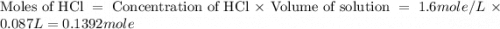
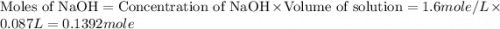

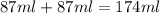
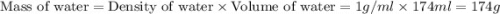
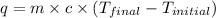
 = specific heat of water =
= specific heat of water = 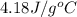
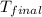 = final temperature of water = 317.4 K
= final temperature of water = 317.4 K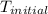 = initial temperature of metal = 298 K
= initial temperature of metal = 298 K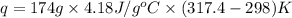
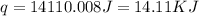
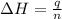
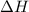 = enthalpy of neutralization = ?
= enthalpy of neutralization = ?