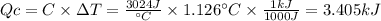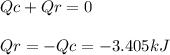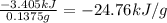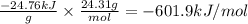Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2


Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
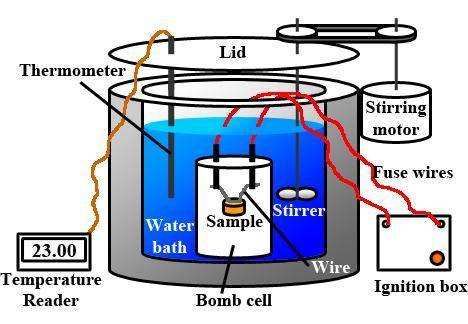
A0.1375-g sample of solid magnesium is burned in a constant-volume bomb calorimeter that has a heat...
Questions

Biology, 21.01.2021 23:00




Mathematics, 21.01.2021 23:00


Mathematics, 21.01.2021 23:00


Mathematics, 21.01.2021 23:00

Mathematics, 21.01.2021 23:00

Mathematics, 21.01.2021 23:00