
Chemistry, 06.07.2019 05:20 baeethtsadia
The wavelength of the red-pink line emitted by a laboratory sample of excited hydrogen is 656 nm. taking a spectrum of a glowing nebula, you find that the same red-pink line of hydrogen appears at 662 nm. you conclude that the nebula
a. is 1% hotter than hydrogen in the laboratory sample.
b. is moving towards us at about 1% the speed of light.
c. is 1% cooler than hydrogen in the laboratory sample.
d. is moving away from us at about 1% the speed of light

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1


Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
The wavelength of the red-pink line emitted by a laboratory sample of excited hydrogen is 656 nm. ta...
Questions






Mathematics, 19.08.2020 16:01


Chemistry, 19.08.2020 16:01

Chemistry, 19.08.2020 16:01

Mathematics, 19.08.2020 16:01


History, 19.08.2020 16:01








Mathematics, 19.08.2020 16:01

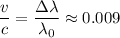 .
.

