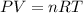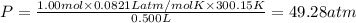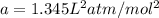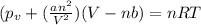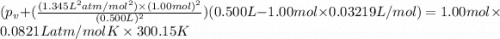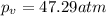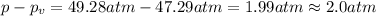Chemistry, 06.07.2019 05:10 ramberson101
If 1.00 mol of argon is placed in a 0.500-l container at 27.0 degree c , what is the difference between the ideal pressure (as predicted by the ideal gas law) and the real pressure (as predicted by the van der waals equation)? for argon, a=1.345(l2⋅atm)/mol2 and b=0.03219l/mol. express your answer to two significant figures and include the appropriate units.

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
If 1.00 mol of argon is placed in a 0.500-l container at 27.0 degree c , what is the difference betw...
Questions


Biology, 02.11.2019 22:31

Mathematics, 02.11.2019 22:31



Arts, 02.11.2019 22:31

Mathematics, 02.11.2019 22:31



Mathematics, 02.11.2019 22:31


Mathematics, 02.11.2019 22:31

English, 02.11.2019 22:31


History, 02.11.2019 22:31

Mathematics, 02.11.2019 22:31

Biology, 02.11.2019 22:31

Biology, 02.11.2019 22:31

History, 02.11.2019 22:31

Mathematics, 02.11.2019 22:31