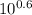Chemistry, 30.01.2020 02:03 lewisf2578
Calculation of original ph from final ph after titration a biochemist has 100 ml of a 0.10 m solution of a weak acid with a pka of 6.3. she adds 6.0 ml of 1.0 m hcl, which changes the ph to 5.7. what was the ph of the original solution?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
Calculation of original ph from final ph after titration a biochemist has 100 ml of a 0.10 m solutio...
Questions

Mathematics, 28.07.2019 11:00





Physics, 28.07.2019 11:00





Mathematics, 28.07.2019 11:00



Mathematics, 28.07.2019 11:00

Social Studies, 28.07.2019 11:00



Mathematics, 28.07.2019 11:00

History, 28.07.2019 11:00

Mathematics, 28.07.2019 11:00