
Chemistry, 06.07.2019 03:20 yazmineespinozarive
Determine the amount of heat (in kj) given off when 1.26 × 104 g of ammonia are produced according to the equation n2(g) + 3h2(g) ⟶ 2nh3(g) δh°rxn = −92.6 kj/mol assume that the reaction takes place under standardstate conditions at 25°c.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2


Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Determine the amount of heat (in kj) given off when 1.26 × 104 g of ammonia are produced according t...
Questions



Health, 27.08.2021 01:00

Computers and Technology, 27.08.2021 01:00

Mathematics, 27.08.2021 01:00

Chemistry, 27.08.2021 01:00


Mathematics, 27.08.2021 01:00

Chemistry, 27.08.2021 01:00

English, 27.08.2021 01:00


Mathematics, 27.08.2021 01:00






Mathematics, 27.08.2021 01:00


Social Studies, 27.08.2021 01:00

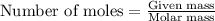
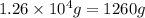
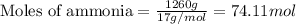
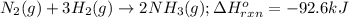
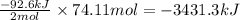 of energy.
of energy.

