Chemistry, 05.07.2019 23:10 rebecabosca
Acylinder with a movable piston contains 2.00 g of helium, he, at room temperature. more helium was added to the cylinder and the volume was adjusted so that the gas pressure remained the same. how many grams of helium were added to the cylinder if the volume was changed from 2.00 l to 4.50 l ? (the temperature was held constant.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

You know the right answer?
Acylinder with a movable piston contains 2.00 g of helium, he, at room temperature. more helium was...
Questions

Biology, 25.09.2021 19:00

Mathematics, 25.09.2021 19:00



English, 25.09.2021 19:00


Mathematics, 25.09.2021 19:00



Mathematics, 25.09.2021 19:10


Health, 25.09.2021 19:10

Chemistry, 25.09.2021 19:10



Chemistry, 25.09.2021 19:10

Spanish, 25.09.2021 19:10

World Languages, 25.09.2021 19:10

Mathematics, 25.09.2021 19:10

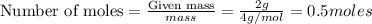
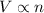 (At constant temperature and pressure)
(At constant temperature and pressure) 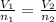
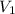 = initial volume of gas = 2.00 L
= initial volume of gas = 2.00 L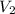 = final volume of gas = 4.50 L
= final volume of gas = 4.50 L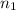 = initial number of moles = 0.5 moles
= initial number of moles = 0.5 moles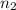 = final number of moles = ?
= final number of moles = ?