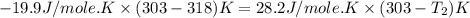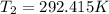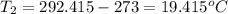Chemistry, 05.07.2019 22:30 itaheart101
Two systems with heat capacities 19.9 j mol-1 k-1 and 28.2 ] mol 1 k-1 respectively interact thermally and come to an equilibrium temperature of 300c. if the initial temperature of system 1 was 450c, what was the initial temperature of system 2 in °c? you may assume that the total energy of the combined systems remains constant

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
Two systems with heat capacities 19.9 j mol-1 k-1 and 28.2 ] mol 1 k-1 respectively interact thermal...
Questions

Mathematics, 03.05.2021 20:30


English, 03.05.2021 20:30

Health, 03.05.2021 20:30

Computers and Technology, 03.05.2021 20:30

Chemistry, 03.05.2021 20:30

Mathematics, 03.05.2021 20:30

Mathematics, 03.05.2021 20:30


Mathematics, 03.05.2021 20:30


Mathematics, 03.05.2021 20:30





Mathematics, 03.05.2021 20:30

History, 03.05.2021 20:30


Mathematics, 03.05.2021 20:30

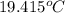
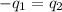
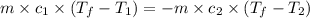
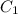 = heat capacity of system 1 = 19.9 J/mole.K
= heat capacity of system 1 = 19.9 J/mole.K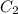 = heat capacity of system 2 = 28.2 J/mole.K
= heat capacity of system 2 = 28.2 J/mole.K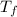 = final temperature of system =
= final temperature of system = 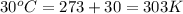
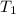 = initial temperature of system 1 =
= initial temperature of system 1 = 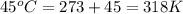
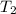 = initial temperature of system 2 = ?
= initial temperature of system 2 = ?