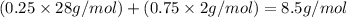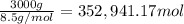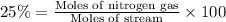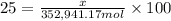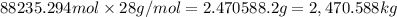Chemistry, 05.07.2019 22:10 tarhondaeiland4122
The feed to an ammonia synthesis reactor contains 25 mole% nitrogen and the balance hydrogen. the fow rate of the stream is 3000 kg/h. calculate the rate of flow of nitrogen into the reactor in kg/h. (suggestion: first calculate the average molecular weight of the mixture.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
The feed to an ammonia synthesis reactor contains 25 mole% nitrogen and the balance hydrogen. the fo...
Questions


Biology, 11.01.2020 16:31

English, 11.01.2020 16:31


Chemistry, 11.01.2020 16:31


Mathematics, 11.01.2020 16:31

Geography, 11.01.2020 16:31



Chemistry, 11.01.2020 16:31

Mathematics, 11.01.2020 16:31

Business, 11.01.2020 16:31




Mathematics, 11.01.2020 16:31

Mathematics, 11.01.2020 16:31

Mathematics, 11.01.2020 16:31