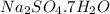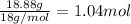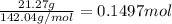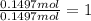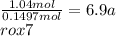Chemistry, 05.07.2019 22:10 thesleepycat
A40.15 gram sample of a hydrate of na2so4 was heated thoroughly in a porcelain crucible, until its weight remained constant. after heating, 21.27 grams of the anhydrous compound remained. what is the formula of the hydrate?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1

Chemistry, 23.06.2019 07:50
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1

Chemistry, 23.06.2019 11:30
Jenny places a strip of ph paper into a solution. when she removes the ph paper, it has turned yellow-green. what should jenny do next to determine the ph of her solution? a. use a different testing method because the ph paper should not change colors b. place the ph paper into a machine that reads the ph of the solution c. compare the ph paper's color with the color of ph paper from another solution d. compare the ph paper's color with a chart of colors and ph ranges
Answers: 1
You know the right answer?
A40.15 gram sample of a hydrate of na2so4 was heated thoroughly in a porcelain crucible, until its w...
Questions

Mathematics, 29.01.2021 14:00

Mathematics, 29.01.2021 14:00

Mathematics, 29.01.2021 14:00

History, 29.01.2021 14:00

Mathematics, 29.01.2021 14:00



Mathematics, 29.01.2021 14:00



Mathematics, 29.01.2021 14:00


Advanced Placement (AP), 29.01.2021 14:00




Physics, 29.01.2021 14:00

Mathematics, 29.01.2021 14:00


Mathematics, 29.01.2021 14:00