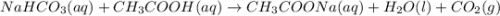Chemistry, 05.07.2019 20:20 jayjinks976
A3.4 g sample of sodium hydrogen carbonate is added to a solution of acetic acid weighing 10.9 g. the two substances react, releasing carbon dioxide gas to the atmosphere. after the reaction, the contents of the reaction vessel weigh 11.6 g. what is the mass of carbon dioxide released during the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2


Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
A3.4 g sample of sodium hydrogen carbonate is added to a solution of acetic acid weighing 10.9 g. th...
Questions





History, 29.06.2019 19:50


English, 29.06.2019 19:50




Biology, 29.06.2019 19:50


Mathematics, 29.06.2019 19:50

Social Studies, 29.06.2019 19:50





Mathematics, 29.06.2019 19:50