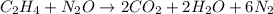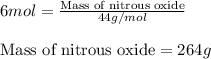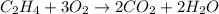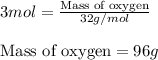Write the balanced chemical equation for the complete, stoichiometric combustion of ethylene in (a) nitrous oxide and (b) air. compare the required number of moles and the oxidizer mass using each of the two oxidizers for the complete, stoichiometric combustion of ethylene.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
Write the balanced chemical equation for the complete, stoichiometric combustion of ethylene in (a)...
Questions

Social Studies, 16.10.2020 17:01

Spanish, 16.10.2020 17:01

English, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01




Mathematics, 16.10.2020 17:01



Chemistry, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

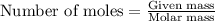 ....(1)
....(1)