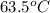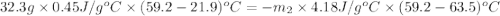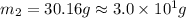Chemistry, 05.07.2019 19:10 stinematesa
A32.2 g iron rod, initially at 21.9 c, is submerged into an unknown mass of water at 63.5 c. in an insulated container. the final temperature of the mixture upon reaching thermal equilibrium is 59 2 c what is the mass of the water? express your answer to two significant figures

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
A32.2 g iron rod, initially at 21.9 c, is submerged into an unknown mass of water at 63.5 c. in an i...
Questions

Chemistry, 03.10.2019 07:30


Mathematics, 03.10.2019 07:30




Social Studies, 03.10.2019 07:30


History, 03.10.2019 07:30

Mathematics, 03.10.2019 07:30

History, 03.10.2019 07:30


Chemistry, 03.10.2019 07:30

World Languages, 03.10.2019 07:30




Biology, 03.10.2019 07:30


Mathematics, 03.10.2019 07:30

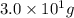
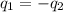
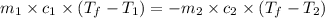
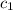 = specific heat of iron metal =
= specific heat of iron metal = 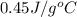
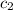 = specific heat of water =
= specific heat of water = 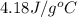
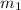 = mass of iron metal = 32.3 g
= mass of iron metal = 32.3 g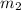 = mass of water = ?
= mass of water = ?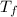 = final temperature of mixture =
= final temperature of mixture = 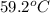
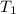 = initial temperature of iron metal =
= initial temperature of iron metal = 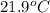
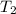 = initial temperature of water =
= initial temperature of water =