
Chemistry, 05.07.2019 06:10 cupkakekawaii45
Consider the following reaction at equilibrium. what effect will adding more so3 have on the system? so2(g) + no2(g) ⇌ so3(g) + no(g) a) the reaction will shift in the direction of products. b) the reaction will shift to decrease the pressure. c) no change will occur since so3 is not included in the equilibrium expression. d) the reaction will shift in the direction of reactants. e) the equilibrium constant will decrease.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:20
An aqueous solution of calcium hydroxide is standardized by titration with a 0.120 m solution of hydrobromic acid. if 16.5 ml of base are required to neutralize 27.5 ml of the acid, what is the molarity of the calcium hydroxide solution?
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Consider the following reaction at equilibrium. what effect will adding more so3 have on the system?...
Questions






English, 23.10.2020 22:00



Biology, 23.10.2020 22:00

English, 23.10.2020 22:00


Mathematics, 23.10.2020 22:00





Mathematics, 23.10.2020 22:00



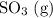 (analog to concentration in a solution.)
(analog to concentration in a solution.) 

