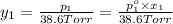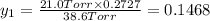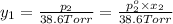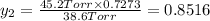Chemistry, 03.07.2019 21:20 mohamedramadan
Asolution contains two isomers, n-propyl alcohol and isopropyl alcohol, at 25°c. the total vapor pressure is 38.6 torr. what are the mole fractions of each alcohol in the liquid and in the vapor phase? the vapor pressures are 21.0 torr for n-propyl alcohol and 45.2 torr for isopropyl alcohol.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Asolution contains two isomers, n-propyl alcohol and isopropyl alcohol, at 25°c. the total vapor pre...
Questions

Computers and Technology, 03.12.2021 18:50


Mathematics, 03.12.2021 18:50


Social Studies, 03.12.2021 18:50





Mathematics, 03.12.2021 18:50

Engineering, 03.12.2021 18:50

Mathematics, 03.12.2021 18:50


Computers and Technology, 03.12.2021 18:50



English, 03.12.2021 18:50

Social Studies, 03.12.2021 18:50


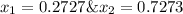 .
.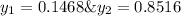 .
.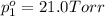
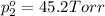
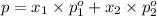 (Raoult's Law)
(Raoult's Law)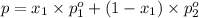
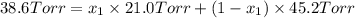
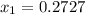
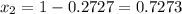
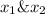 is mole fraction in liquid phase.
is mole fraction in liquid phase.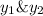
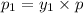 (Dalton's law of partial pressure)
(Dalton's law of partial pressure)