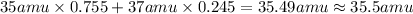The element chlorine (cl) has two isotopes: chlorine‑35 and chlorine‑37. approximately 75.5% of chlorine atoms have 18 neutrons and 17 protons, and the other 24.5% have 20 neutrons and 17 protons. using the isotopic composition provided, calculate the average atomic mass of chlorine. round your answer to the tenths place.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
The element chlorine (cl) has two isotopes: chlorine‑35 and chlorine‑37. approximately 75.5% of chl...
Questions

Mathematics, 10.11.2020 03:50

Mathematics, 10.11.2020 03:50

Advanced Placement (AP), 10.11.2020 03:50


History, 10.11.2020 03:50

Mathematics, 10.11.2020 03:50

Biology, 10.11.2020 03:50


Chemistry, 10.11.2020 03:50

Mathematics, 10.11.2020 03:50

Health, 10.11.2020 03:50

SAT, 10.11.2020 03:50

Mathematics, 10.11.2020 03:50


Chemistry, 10.11.2020 03:50

Mathematics, 10.11.2020 03:50

Mathematics, 10.11.2020 03:50

Chemistry, 10.11.2020 03:50

Mathematics, 10.11.2020 03:50

Mathematics, 10.11.2020 03:50