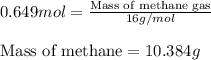Consider the following reaction: 2ch3oh(g) 2ch4(g) + o2(g) δh = +252.8 kj a) calculate the amount of heat transferred when 24.0 g of ch3oh(g) is decomposed by this reaction at constant pressure. b) for a given sample of ch3oh, the enthalpy change during the reaction is 82.1 kj. how many grams of methane gas are produced?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

You know the right answer?
Consider the following reaction: 2ch3oh(g) 2ch4(g) + o2(g) δh = +252.8 kj a) calculate the amount...
Questions









Mathematics, 19.09.2019 21:30








Mathematics, 19.09.2019 21:30



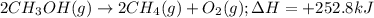
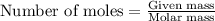 ......(1)
......(1)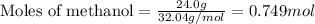
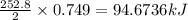
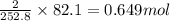 of methane gas is produced.
of methane gas is produced.