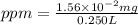Chemistry, 04.02.2020 16:52 kadenreynolds5
A250-ml aqueous solution contains 1.56 mc025-1.jpg 10–5 g of methanol and has a density of 1.03 g/ml. what is the concentration in ppm?
a 6.1 x 10^-8 ppm
b 1.5 x 1-^-5 ppm
c 0.061 ppm
d 15 ppm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
A250-ml aqueous solution contains 1.56 mc025-1.jpg 10–5 g of methanol and has a density of 1.03 g/ml...
Questions




Mathematics, 09.12.2019 18:31

Mathematics, 09.12.2019 18:31

Mathematics, 09.12.2019 18:31





Arts, 09.12.2019 18:31

Mathematics, 09.12.2019 18:31


English, 09.12.2019 18:31

Mathematics, 09.12.2019 18:31

Mathematics, 09.12.2019 18:31




History, 09.12.2019 18:31

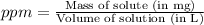
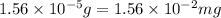 (Conversion Factor : 1g = 1000mg)
(Conversion Factor : 1g = 1000mg)