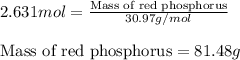Chemistry, 02.07.2019 19:20 fatherbamboo
The reaction between potassium chlorate (kcio,) and red phosphorus (p.) takes place when one strikes a match. the products of the reaction are tetraphosphorus decoxide and potassium chloride. if 56.0 grams of kcio, are reacted with an excess amount of red phosphorus, how many grams of p0o and kci can be produced? how much red phosphorus is consumed in the reaction? (15 pts) write the balanced reaction first!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1

Chemistry, 23.06.2019 08:30
Imagine you are a business executive who wants to pursue an environment policy for your company that limits pollution and uses fewer raw materials but would cost more what might be the discussion to your next broad meeting how would you make your case to your shareholders
Answers: 1
You know the right answer?
The reaction between potassium chlorate (kcio,) and red phosphorus (p.) takes place when one strikes...
Questions


Computers and Technology, 02.09.2019 19:30



English, 02.09.2019 19:30

Mathematics, 02.09.2019 19:30

Computers and Technology, 02.09.2019 19:30

Mathematics, 02.09.2019 19:30

Mathematics, 02.09.2019 19:30

Physics, 02.09.2019 19:30

Mathematics, 02.09.2019 19:30

History, 02.09.2019 19:30

Mathematics, 02.09.2019 19:30







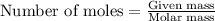 ....(1)
....(1)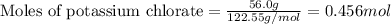
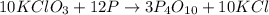
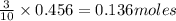 of tetraphosphorus decoxide
of tetraphosphorus decoxide
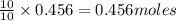 of potassium chloride
of potassium chloride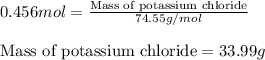
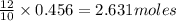 of red phosphorus
of red phosphorus