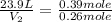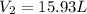Chemistry, 02.07.2019 19:20 azertyqwerty123
Asample of gas contains 0.1300 mol of n2(g) and 0.2600 mol of o2(g) and occupies a volume of 23.9 l. the following reaction takes place: n2(g) + 2o2(g)2no2(g) calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
Asample of gas contains 0.1300 mol of n2(g) and 0.2600 mol of o2(g) and occupies a volume of 23.9 l....
Questions



Mathematics, 01.09.2020 20:01


English, 01.09.2020 20:01


English, 01.09.2020 20:01

Computers and Technology, 01.09.2020 20:01





Mathematics, 01.09.2020 20:01


Mathematics, 01.09.2020 20:01

Computers and Technology, 01.09.2020 20:01



Physics, 01.09.2020 20:01

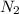 = 0.13 mole
= 0.13 mole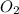 = 0.26 mole
= 0.26 mole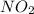 gas.
gas.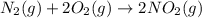
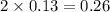 moles of
moles of 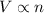
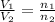
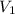 = initial volume of gas = 23.9 L
= initial volume of gas = 23.9 L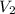 = final volume of gas = ?
= final volume of gas = ?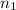 = initial moles of gas = 0.13 + 0.26 = 0.39 mole
= initial moles of gas = 0.13 + 0.26 = 0.39 mole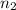 = final moles of gas = 0.26 mole
= final moles of gas = 0.26 mole