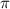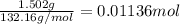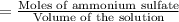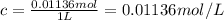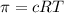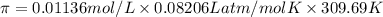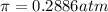Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1


Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2
You know the right answer?
Calculate the osmotic pressure of a solution containing 1.502 g of (nh4)2so4 in 1 l at 36.54 degrees...
Questions



Mathematics, 26.11.2020 01:40

Chemistry, 26.11.2020 01:40


History, 26.11.2020 01:40

Mathematics, 26.11.2020 01:40

Computers and Technology, 26.11.2020 01:40


Mathematics, 26.11.2020 01:40

Chemistry, 26.11.2020 01:40


Biology, 26.11.2020 01:40

Mathematics, 26.11.2020 01:40

Mathematics, 26.11.2020 01:40



Mathematics, 26.11.2020 01:40


Physics, 26.11.2020 01:40