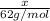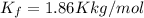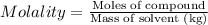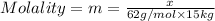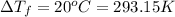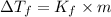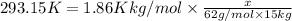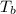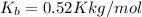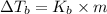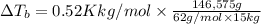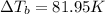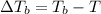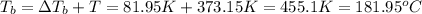Chemistry, 23.10.2019 10:00 jdmXdude3140
Ethylene glycol (c2h4(oh)2) , when dissolved in water, provides the standard ‘anti-freeze’ coolant for water-cooled engines. in order to depress the freezing point of water by 20 °c, how many grams of ethylene glycol would need to be dissolved in 15 kg of pure water? (the molal freezing point depression constant for water kf = 1.86 k mol-1 kg and the relevant atomic masses are: c = 12g, h = 1g and o = 16g.) note: ethylene glycol is an organic compound and does not break up or dissociate when it dissolves in water. hint: first calculate the molality (m) of the ethylene glycol solution.
what is the boiling point of this same solution at atmospheric pressure? (the molal boiling point elevation constant for water kb = 0.52 k mol -1 kg.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
You know the right answer?
Ethylene glycol (c2h4(oh)2) , when dissolved in water, provides the standard ‘anti-freeze’ coolant f...
Questions

Mathematics, 28.01.2020 11:31

History, 28.01.2020 11:31


Biology, 28.01.2020 11:31

Social Studies, 28.01.2020 11:31

History, 28.01.2020 11:31






History, 28.01.2020 11:31

Mathematics, 28.01.2020 11:31


English, 28.01.2020 11:31

History, 28.01.2020 11:31



Spanish, 28.01.2020 11:31