

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 23.06.2019 08:00
Suppose a pair of chemical compounds a and b can react in two different ways: a + b -> c reaction 1 gives product c. a + b -> d reaction 2 gives product d. the following facts are known about the two reactions: . reaction 1 is endothermic and reaction 2 is exothermic. if a reaction vessel is charged (filled) with a and b , then at first d is produced faster than c. use these facts to sketch a qualitative reaction energy diagram for both reactions. note: because these sketches are only qualitative, the energies don? t have to be exact. they only have to have the right relationship to each other. for example, if one energy is less than another, that fact should be clear in your sketch.
Answers: 3
You know the right answer?
Consider the two reactions. 2nh3(g)+3n2o(g)4nh3(g)+3o2(g)⟶4n2(g )+3h2o(l)⟶2n2(g)+6h2o(l) δ∘=−1010 kj...
Questions

English, 18.09.2019 02:40



World Languages, 18.09.2019 02:40

Mathematics, 18.09.2019 02:40

Social Studies, 18.09.2019 02:40




Mathematics, 18.09.2019 02:40


Mathematics, 18.09.2019 02:40

History, 18.09.2019 02:40

History, 18.09.2019 02:40


History, 18.09.2019 02:40


Mathematics, 18.09.2019 02:40


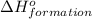 for the reaction is 591.9 kJ.
for the reaction is 591.9 kJ.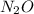 is:
is: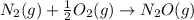
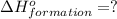
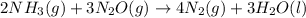
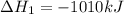 ( ÷ 3)
( ÷ 3)![4NH_3(g)+3O_2(g)\rightarrow 2N_2(g)+6H_2O(l) [tex]\Delta H_2=1531kJ](/tpl/images/0041/0429/f3925.png) ( ÷ 6)
( ÷ 6)![\Delta H^o_{formation}=[\frac{\Delta H_1}{3}]+[\frac{\Delta H_2}{6}]](/tpl/images/0041/0429/5c537.png)
![\Delta H^o_{formation}=[\frac{1010}{3}]+[\frac{1531}{6}]\\\\\Delta H^o_{formation}=591.9kJ](/tpl/images/0041/0429/fba80.png)


