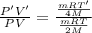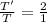Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
Equal volumes of hydrogen and helium gas are at the same pressure. the atomic mass of helium is four...
Questions

Business, 17.10.2019 14:30

History, 17.10.2019 14:30



English, 17.10.2019 14:30



Biology, 17.10.2019 14:30

Spanish, 17.10.2019 14:30

English, 17.10.2019 14:30


Mathematics, 17.10.2019 14:30

Mathematics, 17.10.2019 14:30

Mathematics, 17.10.2019 14:30




Mathematics, 17.10.2019 14:30


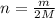
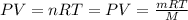 ...(1)
...(1)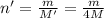
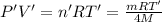 ...(2)
...(2)