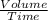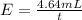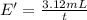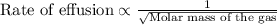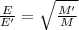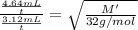Chemistry, 02.07.2019 02:30 slawson4328
Under identical conditions, separate samples of o2 and an unknown gas were allowed to effuse through identical membranes simultaneously. after a certain amount of time, it was found that 4.644.64 ml of o2 had passed through the membrane, but only 3.123.12 ml of of the unknown gas had passed through. what is the molar mass of the unknown gas? unknown molar mass: g/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1
You know the right answer?
Under identical conditions, separate samples of o2 and an unknown gas were allowed to effuse through...
Questions




Law, 27.04.2021 01:00


Mathematics, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00


Chemistry, 27.04.2021 01:00






Social Studies, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00