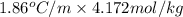Chemistry, 02.07.2019 01:20 lizzyhearts
What is the freezing point of water made by dissolving 22.78 g of ethylene glycol (ch2(oh)ch2(oh)) in 87.95 g of water? the freezing-point depression constant of water is 1.86 oc/m.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2


Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
What is the freezing point of water made by dissolving 22.78 g of ethylene glycol (ch2(oh)ch2(oh)) i...
Questions





Mathematics, 06.10.2019 04:10



Mathematics, 06.10.2019 04:10


Biology, 06.10.2019 04:10

History, 06.10.2019 04:10

Geography, 06.10.2019 04:10

Biology, 06.10.2019 04:10



Computers and Technology, 06.10.2019 04:10



Mathematics, 06.10.2019 04:10

Mathematics, 06.10.2019 04:10

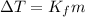
 = change in freezing point
= change in freezing point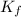 = freezing point depression constant
= freezing point depression constant 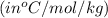
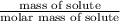
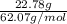
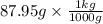 = 0.08795 kg
= 0.08795 kg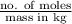
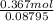
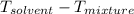 =
= 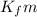
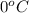 . Now, putting the given values as follows.
. Now, putting the given values as follows.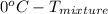 =
=