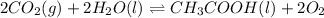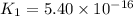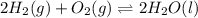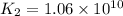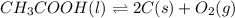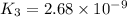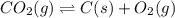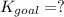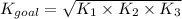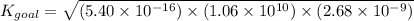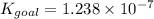Determine the value of the equilibrium constant, kgoal, for the reaction co2(g)⇌c(s)+o2(g), kgoal=? by making use of the following information: 1. 2co2(g)+2h2o(l)⇌ch3cooh(l)+2o2(g), k1 = 5.40×10−16 2. 2h2(g)+o2(g)⇌2h2o(l), k2 = 1.06×1010 3. ch3cooh(l)⇌2c(s)+2h2(g)+o2(g), k3 = 2.68×10−9

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

You know the right answer?
Determine the value of the equilibrium constant, kgoal, for the reaction co2(g)⇌c(s)+o2(g), kgoal=?...
Questions


History, 15.02.2021 17:10

Geography, 15.02.2021 17:10

Mathematics, 15.02.2021 17:10





English, 15.02.2021 17:10


Mathematics, 15.02.2021 17:10

Mathematics, 15.02.2021 17:10


Mathematics, 15.02.2021 17:10



Medicine, 15.02.2021 17:10


Mathematics, 15.02.2021 17:10

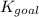 for the final reaction is,
for the final reaction is,