Chemistry, 02.07.2019 00:30 hdjsjfjruejchhehd
Heating 2.40 g of the oxide of metal x (molar mass of x = 55.9 g/mol) in carbon monoxide (co) yields the pure metal and carbon dioxide. the mass of the metal product is 1.68 g. from the data given, show that the simplest formula of the oxide is x2o3 and write a balanced equation for the reaction.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
Heating 2.40 g of the oxide of metal x (molar mass of x = 55.9 g/mol) in carbon monoxide (co) yields...
Questions



History, 09.07.2021 02:30




Mathematics, 09.07.2021 02:30

History, 09.07.2021 02:30

Mathematics, 09.07.2021 02:30







History, 09.07.2021 02:30

History, 09.07.2021 02:30



Mathematics, 09.07.2021 02:30

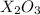 . The balanced equation for the reaction is given by:
. The balanced equation for the reaction is given by: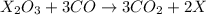
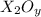
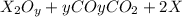
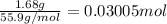
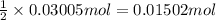 of metal oxide
of metal oxide