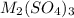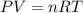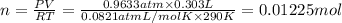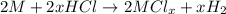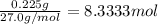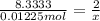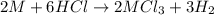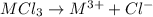Chemistry, 02.07.2019 00:10 naenae6775
Aquantity of 0.225 g of a metal m (molar mass = 27.0 g/mol) liberated 0.303 l of molecular hydrogen (measured at 17°c and 741 mmhg) from an excess of hydrochloric acid. deduce from these data the corresponding equation and write formulas for the oxide and sulfate of m.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
Aquantity of 0.225 g of a metal m (molar mass = 27.0 g/mol) liberated 0.303 l of molecular hydrogen...
Questions


History, 24.04.2020 00:01

English, 24.04.2020 00:02

Biology, 24.04.2020 00:02






Mathematics, 24.04.2020 00:02






History, 24.04.2020 00:02




Mathematics, 24.04.2020 00:10

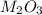 and sulfate of
and sulfate of