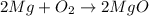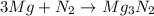Chemistry, 01.07.2019 18:10 jtorres0520
In a common experiment in the general chemistry laboratory, magnesium metal is heated in air to produce mgo. mgo is a white solid, but in these experiments it often looks gray, due to small amounts of mg3n2 , a compound formed as some of the magnesium reacts with nitrogen. write a balanced equation for each reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
In a common experiment in the general chemistry laboratory, magnesium metal is heated in air to prod...
Questions

Medicine, 30.10.2021 15:10

Mathematics, 30.10.2021 15:10




Mathematics, 30.10.2021 15:10


History, 30.10.2021 15:10

Social Studies, 30.10.2021 15:10


Social Studies, 30.10.2021 15:10

Mathematics, 30.10.2021 15:10


Mathematics, 30.10.2021 15:10


Engineering, 30.10.2021 15:10


Mathematics, 30.10.2021 15:10


SAT, 30.10.2021 15:10