Chemistry, 01.07.2019 16:10 richardgibson2005
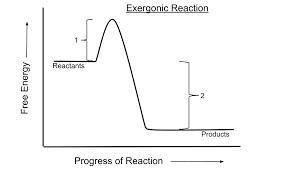
Which of the following is true for all exergonic reactions? the reaction releases energy. a net input of energy from the surroundings is required for the reactions to proceed. the reactions are rapid. the products have more total energy than the reactants. the reaction goes only in a forward direction: all reactants will be converted to products, but no products will be converted to reactants.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

You know the right answer?
Which of the following is true for all exergonic reactions? the reaction releases energy. a net inp...
Questions




Mathematics, 25.10.2020 23:10

Mathematics, 25.10.2020 23:10


English, 25.10.2020 23:10


English, 25.10.2020 23:20


History, 25.10.2020 23:20


Mathematics, 25.10.2020 23:20


English, 25.10.2020 23:20


Mathematics, 25.10.2020 23:20



Mathematics, 25.10.2020 23:20