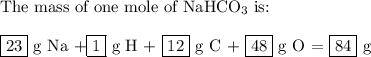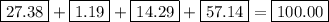Chemistry, 01.07.2019 00:10 lazerlemon500
(need all boxes answered)
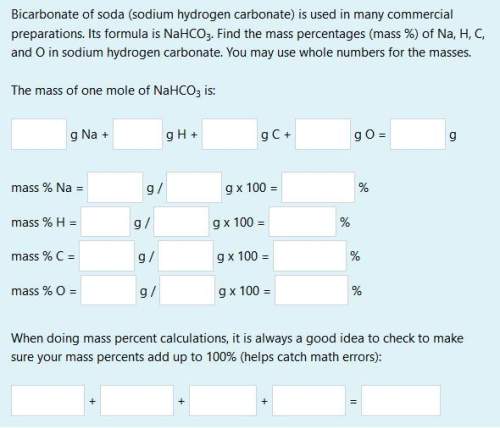
bicarbonate of soda (sodium hydrogen carbonate) is used in many commercial preparations. its formula is nahco3. find the mass percentages (mass %) of na, h, c, and o in sodium hydrogen carbonate. you may use whole numbers for the masses.
the mass of one mole of nahco3 is:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

You know the right answer?
(need all boxes answered)
bicarbonate of soda (sodium hydrogen carbonate) is used in many comm...
bicarbonate of soda (sodium hydrogen carbonate) is used in many comm...
Questions




Mathematics, 19.10.2021 14:00





Chemistry, 19.10.2021 14:00


Mathematics, 19.10.2021 14:00

English, 19.10.2021 14:00

Mathematics, 19.10.2021 14:00

Mathematics, 19.10.2021 14:00



Biology, 19.10.2021 14:00

Mathematics, 19.10.2021 14:00

SAT, 19.10.2021 14:00