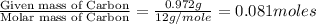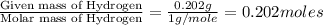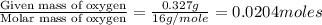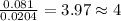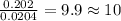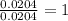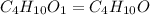The compound known as diethyl ether, commonly referred to as ether, contains carbon, hydrogen, and oxygen. a 1.501 g1.501 g sample of ether was combusted in an oxygen rich environment to produce 3.565 g3.565 g of co2(g)co2(g) and 1.824 g1.824 g of h2o(g)h2o(g) . insert subscripts to complete the empirical formula of ether. empirical formula: cho

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2
You know the right answer?
The compound known as diethyl ether, commonly referred to as ether, contains carbon, hydrogen, and o...
Questions

Social Studies, 10.07.2019 13:00


Mathematics, 10.07.2019 13:00

Mathematics, 10.07.2019 13:00






History, 10.07.2019 13:00

History, 10.07.2019 13:00

History, 10.07.2019 13:00







Social Studies, 10.07.2019 13:00

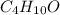
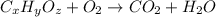
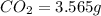
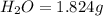
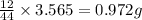 of carbon will be contained.
of carbon will be contained.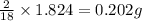 of hydrogen will be contained.
of hydrogen will be contained.