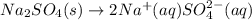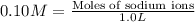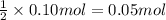Chemistry, 29.06.2019 02:20 SophieStar15
Sodium sulfate dissolves as follows: na2so4(s) → 2na (aq) so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1


Chemistry, 23.06.2019 09:20
Due tomorrow which would have a lower ph, a 0.1 m solution of a strong base or a weak base? why? which would have a higher ph, a 0.1 m solution of a strong base or a weak base? why?
Answers: 3
You know the right answer?
Sodium sulfate dissolves as follows: na2so4(s) → 2na (aq) so42- (aq). how many moles of na2so4 are...
Questions

Biology, 24.05.2020 00:59

Mathematics, 24.05.2020 00:59


Mathematics, 24.05.2020 00:59





Law, 24.05.2020 00:59

History, 24.05.2020 00:59

History, 24.05.2020 00:59

Social Studies, 24.05.2020 00:59




Mathematics, 24.05.2020 01:00

Mathematics, 24.05.2020 01:00

English, 24.05.2020 01:00


Mathematics, 24.05.2020 01:00