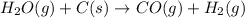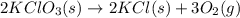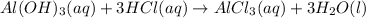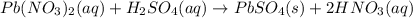Chemistry, 29.06.2019 01:20 ambernolinan
Indicate what type, or types, of reaction each of the following represents: (a) h2 o(g) + c(s) ⟶ co(g) + h2 (g) (b) 2kclo3 (s) ⟶ 2kcl(s) + 3o2 (g) (c) al(oh)3 (aq) + 3hcl(aq) ⟶ alcl3 (aq) + 3h2 o(l) (d) pb(no3 )2 (aq) + h2so4 (aq) ⟶ pbso4 (s) + 2hno3 (aq)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the total reduction potential of a cell in which potassium (k) is reduced and copper (cu) is oxidized? a. 2.59 v b. 3.27 v c. -3.27 v d.-2.59 v
Answers: 1

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
Indicate what type, or types, of reaction each of the following represents: (a) h2 o(g) + c(s) ⟶ co...
Questions

Mathematics, 19.12.2019 08:31

Mathematics, 19.12.2019 08:31

Mathematics, 19.12.2019 08:31

Chemistry, 19.12.2019 08:31

Mathematics, 19.12.2019 08:31

Biology, 19.12.2019 08:31



Mathematics, 19.12.2019 08:31


Mathematics, 19.12.2019 08:31

Mathematics, 19.12.2019 08:31

History, 19.12.2019 08:31

Mathematics, 19.12.2019 08:31

History, 19.12.2019 08:31





Mathematics, 19.12.2019 08:31