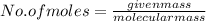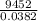Chemistry, 29.06.2019 00:20 webbjalia04
A2.50 g sample of powdered zinc is added to 100.0 ml of a 2.00 m aqueous solution of hydrobromic acid in a calorimeter. the total heat capacity of the calorimeter and solution is 448 j/k. the observed increase in temperature is 21.1 k at a constant pressure of one bar. calculate the standard enthalpy of reaction using these data. zn(s)+2hbr(aq)⟶znbr2(aq)+h2(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
A2.50 g sample of powdered zinc is added to 100.0 ml of a 2.00 m aqueous solution of hydrobromic aci...
Questions

Mathematics, 18.10.2019 10:50



Business, 18.10.2019 10:50


Geography, 18.10.2019 10:50

Health, 18.10.2019 10:50


Mathematics, 18.10.2019 10:50

History, 18.10.2019 10:50






Mathematics, 18.10.2019 10:50


Mathematics, 18.10.2019 10:50