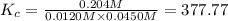Carbonyl chloride (cocl2), also called phosgene, was used in world war i as a poisonous gas. the equilibrium concentrations for the reaction between carbon monoxide and molecular chlorine to form carbonyl chloride at a certain temperature are [co] = 0.0210 m, [cl2] = 0.0450 m, and [cocl2] = 0.204 m. co(g) + cl2(g) ⇆ cocl2(g) calculate the equilibrium constant (kc).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1


Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

You know the right answer?
Carbonyl chloride (cocl2), also called phosgene, was used in world war i as a poisonous gas. the equ...
Questions

Mathematics, 15.12.2020 21:30



Biology, 15.12.2020 21:30



Advanced Placement (AP), 15.12.2020 21:30


Mathematics, 15.12.2020 21:30

Mathematics, 15.12.2020 21:30

Mathematics, 15.12.2020 21:30



Computers and Technology, 15.12.2020 21:30

Mathematics, 15.12.2020 21:30

Business, 15.12.2020 21:30


Mathematics, 15.12.2020 21:30

Mathematics, 15.12.2020 21:30

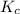
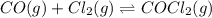
![[CO] = 0.0210 M](/tpl/images/0028/7944/23f4d.png)
![[Cl_2] = 0.0450 M](/tpl/images/0028/7944/558aa.png)
![[COCl_2] = 0.204 M](/tpl/images/0028/7944/1309b.png)
![K_c=\frac{[COCl_2]}{[CO][Cl_2]}](/tpl/images/0028/7944/36d91.png)