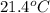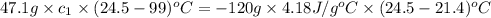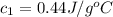Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Metallic bonds are good conductors of electricity true or false
Answers: 2

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2
You know the right answer?
A47.1 g sample of a metal is heated to 99.0°c and then placed in a calorimeter containing 120.0 g of...
Questions


Mathematics, 21.11.2020 03:00


Mathematics, 21.11.2020 03:00

Mathematics, 21.11.2020 03:00

Chemistry, 21.11.2020 03:00

English, 21.11.2020 03:00

Mathematics, 21.11.2020 03:00

Mathematics, 21.11.2020 03:00


Mathematics, 21.11.2020 03:00




English, 21.11.2020 03:00

Mathematics, 21.11.2020 03:00

Mathematics, 21.11.2020 03:00

Mathematics, 21.11.2020 03:00

Mathematics, 21.11.2020 03:00

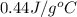 ).
).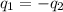
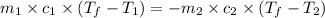
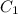 = specific heat of metal = ?
= specific heat of metal = ?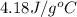
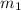 = mass of metal = 47.1 g
= mass of metal = 47.1 g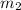 = mass of water = 120 g
= mass of water = 120 g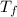 = final temperature of water =
= final temperature of water = 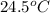
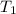 = initial temperature of metal =
= initial temperature of metal = 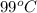
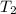 = initial temperature of water =
= initial temperature of water =