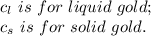Chemistry, 28.06.2019 18:30 ilizzy1224
2.0 kg of solid gold (au) at an initial temperature of 1000k is allowed to exchange heat with 1.5 kg of liquid gold at an initial temperature at 1336k. the solid and liquid other. when the two reach thermal equilibrium will the mixture be entirely solid, or will they be in a mixed solid/liquid phase? explain how you know. draw two separate temp. vs. energy added diagrams to you answer this question. can only exchange heat with each

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
2.0 kg of solid gold (au) at an initial temperature of 1000k is allowed to exchange heat with 1.5 kg...
Questions

Mathematics, 21.11.2020 04:10



Mathematics, 21.11.2020 04:10

English, 21.11.2020 04:10


Medicine, 21.11.2020 04:10

History, 21.11.2020 04:10

Biology, 21.11.2020 04:10

History, 21.11.2020 04:10




English, 21.11.2020 04:20



English, 21.11.2020 04:20

Chemistry, 21.11.2020 04:20

Advanced Placement (AP), 21.11.2020 04:20