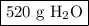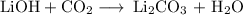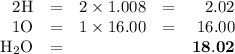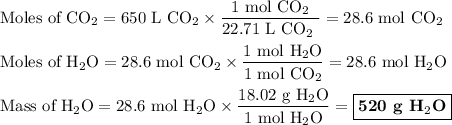Chemistry, 28.06.2019 04:10 lilymessina94
Answer the following question: in a space shuttle, the co2 that the crew exhales is removed from the air by a reaction within canisters of lithium hydroxide. on average, each astronaut exhales about 650 l of co2 daily. what mass of water will be produced
when this amount reacts with lioh? the other product of the reaction is
li2co3. when answering this question include the following:
have both the unbalanced and balanced chemical equations.
explain how to find the molar mass of the compounds.
explain how the balanced chemical equation is used to find the ratio of moles (hint: step 3 in the video).
explain how many significant figures your answer needs to have.
the numerical answer

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 23:30
What mass of propane is necessary to react with the amount of oxygen calculated in the previous question? g c3h8
Answers: 1
You know the right answer?
Answer the following question: in a space shuttle, the co2 that the crew exhales is removed from th...
Questions

English, 22.04.2021 21:30

Mathematics, 22.04.2021 21:30


Mathematics, 22.04.2021 21:30

Social Studies, 22.04.2021 21:30


Mathematics, 22.04.2021 21:30

Mathematics, 22.04.2021 21:30

Mathematics, 22.04.2021 21:30

Mathematics, 22.04.2021 21:30


Mathematics, 22.04.2021 21:30



History, 22.04.2021 21:30




Mathematics, 22.04.2021 21:30

World Languages, 22.04.2021 21:30