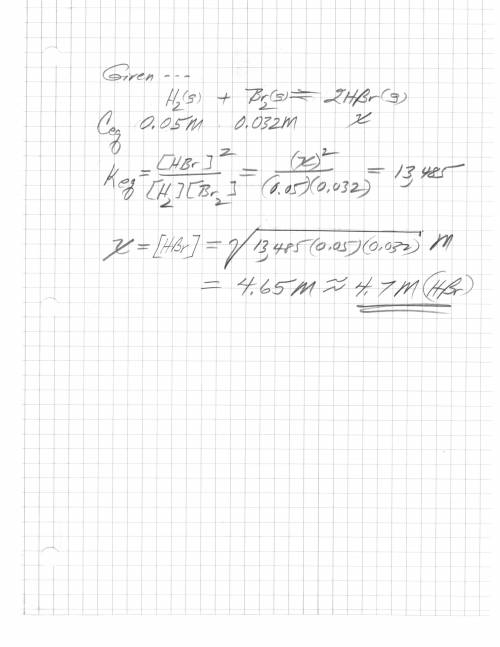
H2 (g) + br2 (g) < => 2 hbr (g)
the equilibrium constant is 13485. at equilibrium...

Chemistry, 23.11.2019 20:31 abronxtale02
H2 (g) + br2 (g) < => 2 hbr (g)
the equilibrium constant is 13485. at equilibrium the h2 concentration is 0.05 m, while the br2 concentration is 0.023 m. calculate the hbr concentration at equilibrium, to 1 decimal. be careful with the units.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

You know the right answer?
Questions

Chemistry, 13.11.2020 21:00




Mathematics, 13.11.2020 21:00

History, 13.11.2020 21:00


Mathematics, 13.11.2020 21:00

Mathematics, 13.11.2020 21:00


Mathematics, 13.11.2020 21:00


Biology, 13.11.2020 21:00