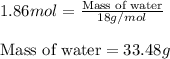Chemistry, 27.06.2019 19:30 jeffmacdonald1976
Given the following equation: |4 nh3 (g) + 5 о2 (g) —> 4 no (g) + 6 h20 () how many grams of h20 is produced if 21.1 grams of nh3 reacts with 73.9 grams of o2?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
Given the following equation: |4 nh3 (g) + 5 о2 (g) —> 4 no (g) + 6 h20 () how many grams of h2...
Questions


History, 27.11.2020 05:30





Business, 27.11.2020 05:30

English, 27.11.2020 05:30


Chemistry, 27.11.2020 05:30


Mathematics, 27.11.2020 05:30

Physics, 27.11.2020 05:30

Mathematics, 27.11.2020 05:30

Advanced Placement (AP), 27.11.2020 05:30

History, 27.11.2020 05:30




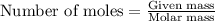 ....(1)
....(1)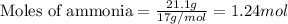
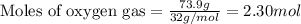
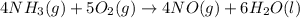
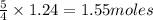 of oxygen gas.
of oxygen gas.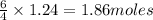 of water.
of water.