

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Given the following equation: 4 nh3 (g)5 o2 (g) > 4 no (g) 6 h20 () + how many moles of h20 is...
Questions





History, 25.02.2021 21:10

History, 25.02.2021 21:10

Social Studies, 25.02.2021 21:10

Mathematics, 25.02.2021 21:10




Mathematics, 25.02.2021 21:10


Chemistry, 25.02.2021 21:10

Mathematics, 25.02.2021 21:10

Mathematics, 25.02.2021 21:10

History, 25.02.2021 21:10

Mathematics, 25.02.2021 21:20

Mathematics, 25.02.2021 21:20

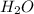 produced are, 0.66 mole.
produced are, 0.66 mole.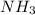 = 0.44 mole
= 0.44 mole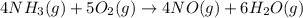
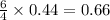 moles of
moles of 

