
Chemistry, 27.06.2019 03:20 xnadertheking
The value of δh° for the reaction below is -126 kj. the amount of heat that is released by the reaction of 10.0 g of na2o2 with water is kj. 2na2o2 (s) + 2h2o (l) → 4naoh (s) + o2 (g)

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 14:30
Which statement justifies that phosphine (ph3) is a polar molecule?
Answers: 1

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3
You know the right answer?
The value of δh° for the reaction below is -126 kj. the amount of heat that is released by the react...
Questions

Mathematics, 17.07.2019 05:30

Computers and Technology, 17.07.2019 05:30


Mathematics, 17.07.2019 05:30



History, 17.07.2019 05:30


History, 17.07.2019 05:30







Mathematics, 17.07.2019 05:30

Mathematics, 17.07.2019 05:30



Mathematics, 17.07.2019 05:30

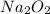 will be -8.064 kJ.
will be -8.064 kJ.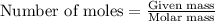
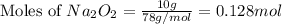
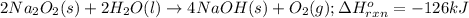
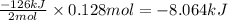 of energy.
of energy.

