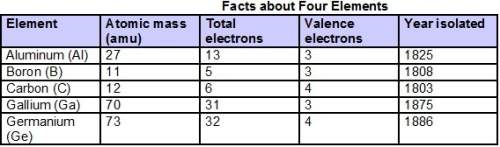
Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3


Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1
You know the right answer?
Asample of neon has a volume of 5.00 l, and initial temperature of 425 k, and an initial pressure of...
Questions


Mathematics, 25.08.2019 15:00

Geography, 25.08.2019 15:00


History, 25.08.2019 15:00

Geography, 25.08.2019 15:00

Social Studies, 25.08.2019 15:00


Biology, 25.08.2019 15:00


Biology, 25.08.2019 15:00


English, 25.08.2019 15:00



Computers and Technology, 25.08.2019 15:00

Arts, 25.08.2019 15:00

English, 25.08.2019 15:00


Biology, 25.08.2019 15:00