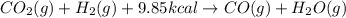Chemistry, 26.06.2019 20:20 Santos7446
When co2(g) reacts with h2(g) to form co(g) and h2o(g) , 9.85 kcal of energy are absorbed for each mole of co2(g) that reacts. write a balanced equation for the reaction with an energy term in kcal as part of the equation.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2


Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
You know the right answer?
When co2(g) reacts with h2(g) to form co(g) and h2o(g) , 9.85 kcal of energy are absorbed for each m...
Questions


Social Studies, 08.12.2020 16:30




English, 08.12.2020 16:30

Mathematics, 08.12.2020 16:30




Social Studies, 08.12.2020 16:30


Mathematics, 08.12.2020 16:30


Mathematics, 08.12.2020 16:30

Chemistry, 08.12.2020 16:30

Business, 08.12.2020 16:30

History, 08.12.2020 16:30