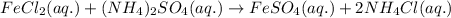Chemistry, 28.01.2020 20:07 kitttimothy55
Consider the reaction. fecl2(aq)+(nh4)2 so4(aq)⟶feso4+2nh4cl identify the precipitate, or lack thereof, for the reaction. (a) feso4 (b) no precipitate (c) nh4cl

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Consider the reaction. fecl2(aq)+(nh4)2 so4(aq)⟶feso4+2nh4cl identify the precipitate, or lack there...
Questions

Chemistry, 03.11.2020 17:30

Mathematics, 03.11.2020 17:30







Mathematics, 03.11.2020 17:30



Physics, 03.11.2020 17:30


Spanish, 03.11.2020 17:30

Mathematics, 03.11.2020 17:30