
Chemistry, 26.06.2019 16:10 pchisholm100
Aluminum chloride can be formed from its elements:
(i) 2al(s) + 3cl2 (g) ⟶ 2alcl3 (s) δh° = ? use the reactions here to determine the δh° for reaction (i):
(ii) hcl(g) ⟶ hcl(aq) δh(ii) ° = −74.8 kj
(iii) h2 (g) + cl2 (g) ⟶ 2hcl(g) δh(iii) ° = −185 kj
(iv) alcl3 (aq) ⟶ alcl3 (s) δh(iv) ° = +323 kj/mol
(v) 2al(s) + 6hcl(aq) ⟶ 2alcl3 (aq) + 3h2 (g) δh(v) ° = −1049 kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 1
You know the right answer?
Aluminum chloride can be formed from its elements:
(i) 2al(s) + 3cl2 (g) ⟶ 2alcl3 (s) δh° =...
(i) 2al(s) + 3cl2 (g) ⟶ 2alcl3 (s) δh° =...
Questions

History, 04.11.2020 20:10


Mathematics, 04.11.2020 20:10

History, 04.11.2020 20:10

Mathematics, 04.11.2020 20:10



Physics, 04.11.2020 20:10

Mathematics, 04.11.2020 20:10





Health, 04.11.2020 20:10

Mathematics, 04.11.2020 20:10



Advanced Placement (AP), 04.11.2020 20:10

English, 04.11.2020 20:10

Mathematics, 04.11.2020 20:10

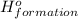 for the reaction is -1406.8 kJ.
for the reaction is -1406.8 kJ.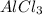 is:
is: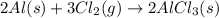
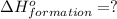
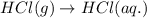
 ( × 6)
( × 6)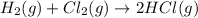
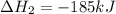 ( × 3)
( × 3)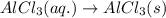
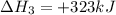 ( × 2)
( × 2)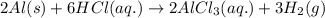
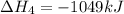
![\Delta H^o_{formation}=[6\times \Delta H_1]+[3\times \Delta H_2]+[2\times \Delta H_3]+[1\times \Delta H_4]](/tpl/images/0020/0754/79ecd.png)
![\Delta H^o_{formation}=[(-74.8\times 6)+(-185\times 3)+(323\times 2)+(-1049\times 1)]=-1406.8kJ](/tpl/images/0020/0754/37adf.png)


