
Chemistry, 26.06.2019 05:20 sindy35111
The combustion of ethane (c2h6)(c2h6) produces carbon dioxide and steam. 2c2h6(g)+7o2(g)⟶4co2(g)+6h2o(g) 2c2h6(g)+7o2(g)⟶4co2(g)+6h2o(g) how many moles of co2co2 are produced when 5.95 mol5.95 mol of ethane is burned in an excess of oxygen? moles of co2: co2:

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1
You know the right answer?
The combustion of ethane (c2h6)(c2h6) produces carbon dioxide and steam. 2c2h6(g)+7o2(g)⟶4co2(g)+6h2...
Questions


Mathematics, 25.06.2019 19:30

Computers and Technology, 25.06.2019 19:30

Mathematics, 25.06.2019 19:30

Geography, 25.06.2019 19:30


Mathematics, 25.06.2019 19:30



Chemistry, 25.06.2019 19:30




English, 25.06.2019 19:30



Physics, 25.06.2019 19:30

Mathematics, 25.06.2019 19:30


History, 25.06.2019 19:30


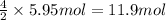 of carbon-dioxide
of carbon-dioxide

